Therapy development
“Those affected have no time to lose – especially as sufficient preliminary work has already been carried out at the country’s most research-intensive clinics to get promising therapies off the ground.”
– Prof. Dr. Annette Grüters-Kieslich
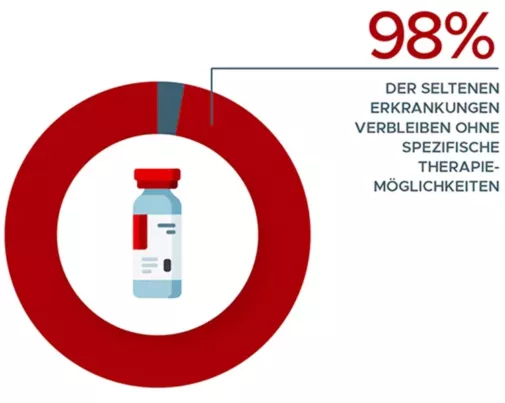
Orphan Drugs
Specific drugs are only available for just over 2% of all rare diseases. There are currently 151 orphan drugs approved in the EU. For a further 83 drugs, the orphan drug status was either withdrawn after approval or expired after 10 years.
These include many cancer drugs, as many tumors are classified as rare diseases due to their prevalence of less than 5 in 10,000 people in the EU. The ratio is significantly worse for all other specialist areas.
Due to the small number of cases, the clinical studies required for the approval of a drug are so complex and expensive that the usual market mechanisms do not work. The sad truth: rare diseases are not worthwhile for pharmaceutical companies.
Until 1999, there was virtually no market for orphan drugs in Germany. An important milestone for patients was the EU regulation on orphan drugs in 2000, which enabled a simplified approval procedure for orphan drugs and granted manufacturers exclusive rights for a limited period of time.
Did you know, that...

Rising drug approval rates – financial constraints
Because some orphan drugs are particularly expensive, discussions about pricing and access to these medicines have been increasing since the number of approvals has also risen in Germany.
Our healthcare system, which is financed on the basis of solidarity, is faced with a variety of challenges and must keep an eye on the costs for the insured community. However, orphan drugs only accounted for around 4.4 percent of outpatient drug expenditure in statutory health insurance in 2020.
The fact is: Causative treatments are only available for a small fraction of rare diseases. Targeted investment in the further development of promising therapeutic approaches can change this.

Help the orphans of medicine!
ELHKS uses your donations in a targeted manner – so that medical progress reaches everyone.

Innovative procedures: A turning point in therapy
Today, researchers are increasingly succeeding in deciphering the pathological mechanisms of disease development. This provides starting points for the development of targeted drugs and antibodies that can significantly reduce the symptoms of affected people.
Encouraging treatment successes with gene and cell therapy methods are heralding a real turning point in medicine. In particular, those affected by rare diseases with a monogenetic cause could hope for a cure if promising research approaches were now consistently pursued.
In order to drive forward the development of promising treatment approaches, the Alliance4Rare initiated by our foundation pools the expertise of research-intensive university children’s hospitals along a cross-location research strategy for rare diseases.

Opportunity or risk?
There is not yet sufficient long-term experience with the methods presented. However, they are used for diseases that lead to death in childhood or are associated with particularly severe developmental restrictions if left untreated. Experience has shown that the uncertain long-term risk is more acceptable for those affected without an alternative therapy than the devastating short to medium-term prognosis.
The way to optimize the long-term risk-benefit ratio is clear:
1. clinical studies on the efficacy, tolerability and practicability of therapeutic approaches
2. data-based recording and evaluation of the improvement in quality of life
3. systematic long-term observations to clarify possible long-term consequences for children and adolescents
Consistent research funding makes a decisive contribution to minimizing the risks for those affected and sustainably increasing their chances of survival and quality of life.
Newsletter Subscription
Our free newsletter informs you about current calls for proposals, events and projects concerning rare diseases.